Back ناقل الحديد Arabic Transferrina Catalan Transferin Czech TF Welsh Transferrin German Transferrin English Transferrina Spanish ترانسفرین Persian Transferrine French Transferrina Galician
| Transferin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifikatori | |||||||||
| Simbol | TF | ||||||||
| Pfam | PF00405 | ||||||||
| InterPro | IPR001156 | ||||||||
| PROSITE | PDOC00182 | ||||||||
| SCOP2 | 1lcf / SCOPe / SUPFAM | ||||||||
| |||||||||
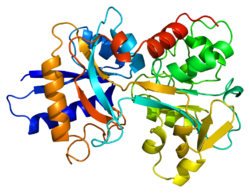
Transferini su glikoproteini koji se nalaze u kičmenjacima, a vezuju se za i posljedično posreduju u transportu gvožđa (Fe) kroz krvnu plazmu.[5] Proizvode se u jetri i sadrže mjesta vezivanja za dva iona Fe3+.[6] Ljudski transferin je kodiran genom TF i proizveden je kao glikoproten od 76 kDa.[7][8]
Transferinski glikoprotein vezuje gvožđe čvrsto, ali reverzibilno. Iako je gvožđe vezano za transferin manje od 0,1% (4 mg) ukupnog gvožđa u tijelu, ono čini najvitalniji bazen gvožđa sa najvećom stopom obrtanja (25 mg/24 h). Transferin ima molekulsku težinu od oko 80 kDa i sadrži dva specifična mjesta visokog afiniteta Fe(III) vezivanja. Afinitet transferina prema Fe(III) je izuzetno visok (konstanta disocijacije je 1020 M−1 na pH 7.4)[9] ali se progresivno smanjuje sa smanjenjem pH ispod neutralnosti. Transferini nisu ograničeni samo na vezivanje za gvožđe, već i za različite ione metala.[10] Ovi glikoproteini nalaze se u različitim tjelesnim tekućinama kičmenjaka.[11][12] Neki beskičmenjaci imaju proteine koji se ponašaju kao transferin koji se nalazi u hemolimfi.[11][13]
Kada nije vezan za željezo, transferin je poznat kao "apotransferin" (vidi također Apoprotein).
- ^ a b c GRCh38: Ensembl release 89: ENSG00000091513 - Ensembl, maj 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000032554 - Ensembl, maj 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Crichton RR, Charloteaux-Wauters M (maj 1987). "Iron transport and storage". European Journal of Biochemistry. 164 (3): 485–506. doi:10.1111/j.1432-1033.1987.tb11155.x. PMID 3032619.
- ^ Hall DR, Hadden JM, Leonard GA, Bailey S, Neu M, Winn M, Lindley PF (januar 2002). "The crystal and molecular structures of diferric porcine and rabbit serum transferrins at resolutions of 2.15 and 2.60 A, respectively". Acta Crystallographica. Section D, Biological Crystallography. 58 (Pt 1): 70–80. doi:10.1107/s0907444901017309. PMID 11752780.
- ^ Yang F, Lum JB, McGill JR, Moore CM, Naylor SL, van Bragt PH, et al. (maj 1984). "Human transferrin: cDNA characterization and chromosomal localization". Proceedings of the National Academy of Sciences of the United States of America. 81 (9): 2752–6. Bibcode:1984PNAS...81.2752Y. doi:10.1073/pnas.81.9.2752. PMC 345148. PMID 6585826.
- ^ Kawabata H (mart 2019). "Transferrin and transferrin receptors update". Free Radical Biology & Medicine. 133: 46–54. doi:10.1016/j.freeradbiomed.2018.06.037. PMID 29969719. S2CID 49674402.
- ^ Aisen P, Leibman A, Zweier J (mart 1978). "Stoichiometric and site characteristics of the binding of iron to human transferrin". The Journal of Biological Chemistry. 253 (6): 1930–7. doi:10.1016/S0021-9258(19)62337-9. PMID 204636.
- ^ Nicotra S, Sorio D, Filippi G, De Gioia L, Paterlini V, De Palo EF, et al. (novembar 2017). "Terbium chelation, a specific fluorescent tagging of human transferrin. Optimization of conditions in view of its application to the HPLC analysis of carbohydrate-deficient transferrin (CDT)". Analytical and Bioanalytical Chemistry. 409 (28): 6605–6612. doi:10.1007/s00216-017-0616-z. PMID 28971232. S2CID 13929228.
- ^ a b MacGillivray RT, Moore SA, Chen J, Anderson BF, Baker H, Luo Y, et al. (juni 1998). "Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release". Biochemistry. 37 (22): 7919–28. doi:10.1021/bi980355j. PMID 9609685.
- ^ Dewan JC, Mikami B, Hirose M, Sacchettini JC (novembar 1993). "Structural evidence for a pH-sensitive dilysine trigger in the hen ovotransferrin N-lobe: implications for transferrin iron release". Biochemistry. 32 (45): 11963–8. doi:10.1021/bi00096a004. PMID 8218271.
- ^ Baker EN, Lindley PF (august 1992). "New perspectives on the structure and function of transferrins". Journal of Inorganic Biochemistry. 47 (3–4): 147–60. doi:10.1016/0162-0134(92)84061-q. PMID 1431877.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search